
Exhibit 99.1

CORPORATE OVERVIEW NASDAQ : FBIO October 2016

FORWARD - LOOKING STATEMENTS Statements in this presentation that are not descriptions of historical facts are forward - looking statements within the meaning of the “ safe harbor ” provisions of the Private Securities Litigation Reform Act of 1995 . We have attempted to identify forward - looking statements by terminology including “ anticipates, ” “ believes, ” “ can, ” “ continue, ” “ could, ” “ estimates, ” “ expects, ” “ intends, ” “ may, ” “ plans, ” “ potential, ” “ predicts, ” “ should, ” or “ will ” or the negative of these terms or other comparable terminology . Forward - looking statements are based on management ’ s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price . Factors that could cause actual results to differ materially from those currently anticipated are risks relating to : our growth strategy ; results of research and development activities ; uncertainties relating to preclinical and clinical testing ; our dependence on third party suppliers ; our ability to obtain, perform under and maintain financing and strategic agreements and relationships ; our ability to attract, integrate, and retain key personnel ; the early stage of products under development ; our need for substantial funds ; government regulation ; patent and intellectual property matters ; competition ; as well as other risks described in our SEC filings . We expressly disclaim any obligation or undertaking to update or revise any statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances after the date of this presentation . 2
FORTRESS BIOTECH COMPANIES Acute settings (pain) Multiple cancers/Epigene tics/Antibodies Orphan diseases Infectious diseases (CMV) Dermatology (marketed products) CAR - T/ Immuno - oncology (brain cancer, leukemia) 3
OVERVIEW & BUSINESS STRATEGY Acquire, develop and commercialize a diversified portfolio of products directly and through subsidiary companies (known as “Fortress Companies”) FBIO holds super - majority voting shares of each Fortress Company Multiple sources of return for FBIO, direct sales, royalties, equity stakes and service fees 4
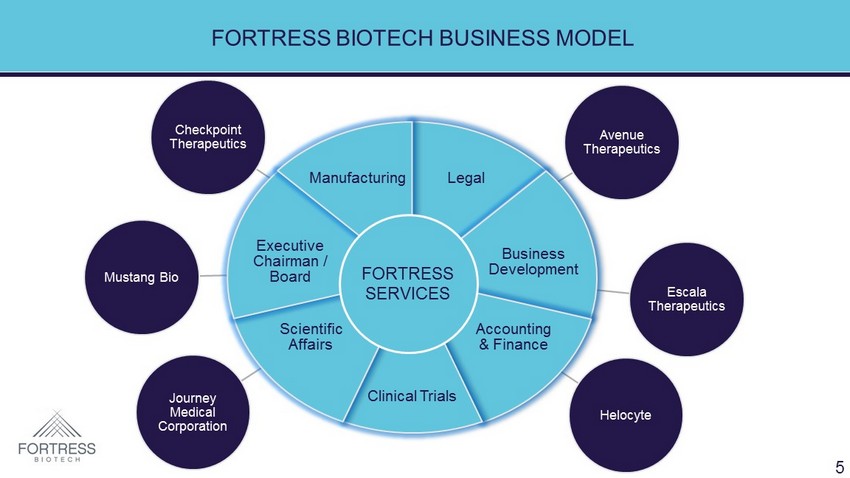
Checkpoint Therapeutics Avenue Therapeutics Escala Therapeutics Helocyte Journey Medical Corporation Mustang Bio Legal Business Development Accounting & Finance Clinical Trials Scientific Affairs Executive Chairman / Board Manufacturing FORTRESS BIOTECH BUSINESS MODEL FORTRESS SERVICES 5

• CAR - T Program Including Solid Tumor (GBM) Complete Response • CMV Vaccine Program: In 2 Phase 2 Clinical Trials: Positive randomized trial recently published in the Lancet Hematology • Internal Immuno - Oncology program makes Fortress one of the only Life Sciences Companies with its own internal I/O and CAR - T Programs • Other Phase 2 and Phase 3 Programs soon to launch • A business development engine with 15 full time B/D staff and growing VALUE PROPOSITION IN JUST 2 YEARS, FORTRESS HAS ACCOMPLISHED: 6
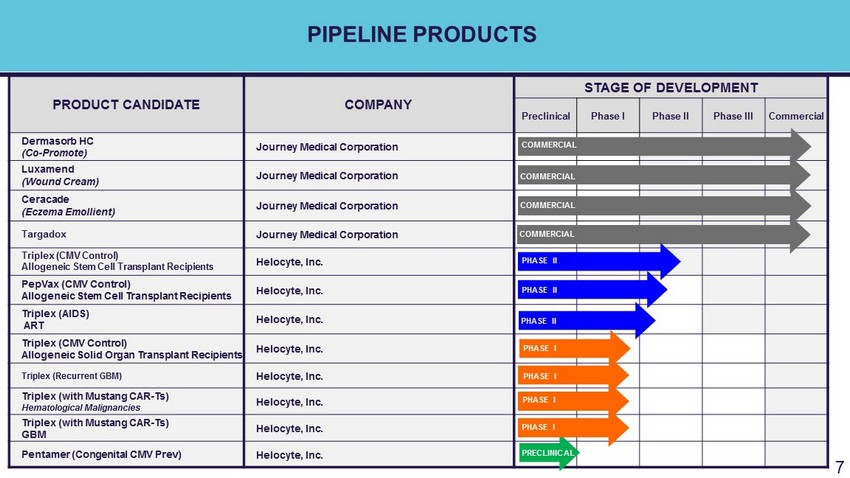
PIPELINE PRODUCTS PRODUCT CANDIDATE COMPANY STAGE OF DEVELOPMENT Preclinical Phase I Phase II Phase III Commercial Dermasorb HC (Co - Promote) Journey Medical Corporation Luxamend (Wound Cream) Journey Medical Corporation Ceracade (Eczema Emollient) Journey Medical Corporation Targadox Journey Medical Corporation Triplex (CMV Control) Allogeneic Stem Cell Transplant Recipients Helocyte, Inc. PepVax (CMV Control) Allogeneic Stem Cell Transplant Recipients Helocyte, Inc. PHASE II Triplex (AIDS) ART Helocyte, Inc. PHASE II Triplex (CMV Control) Allogeneic Solid Organ Transplant Recipients Helocyte, Inc. Triplex (Recurrent GBM) Helocyte, Inc. Triplex (with Mustang CAR - Ts) Hematological Malignancies Helocyte, Inc. Triplex (with Mustang CAR - Ts) GBM Helocyte, Inc. Pentamer (Congenital CMV Prev ) Helocyte, Inc. 7 COMMERCIAL COMMERCIAL COMMERCIAL COMMERCIAL PHASE II PHASE II PHASE II PRECLINICAL PHASE I PHASE I PHASE I PHASE I
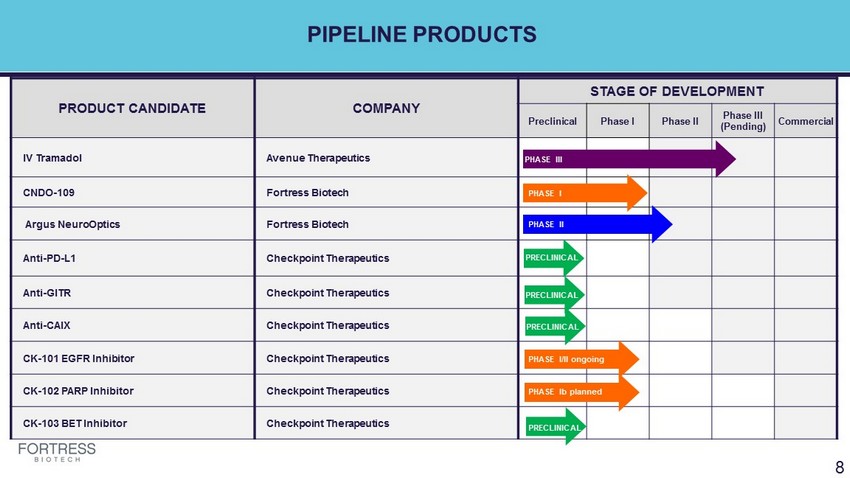
PIPELINE PRODUCTS PRODUCT CANDIDATE COMPANY STAGE OF DEVELOPMENT Preclinical Phase I Phase II Phase III (Pending) Commercial IV Tramadol Avenue Therapeutics CNDO - 109 Fortress Biotech Argus NeuroOptics Fortress Biotech Anti - PD - L1 Checkpoint Therapeutics Anti - GITR Checkpoint Therapeutics Anti - CAIX Checkpoint Therapeutics PHASE II CK - 101 EGFR Inhibitor Checkpoint Therapeutics PHASE II CK - 102 PARP Inhibitor Checkpoint Therapeutics CK - 103 BET Inhibitor Checkpoint Therapeutics 8 PHASE III PHASE I PHASE II PHASE I PHASE I PHASE I/II ongoing PHASE I PHASE Ib planned PHASE I PRECLINICAL PRECLINICAL PRECLINICAL PRECLINICAL PRECLINICAL
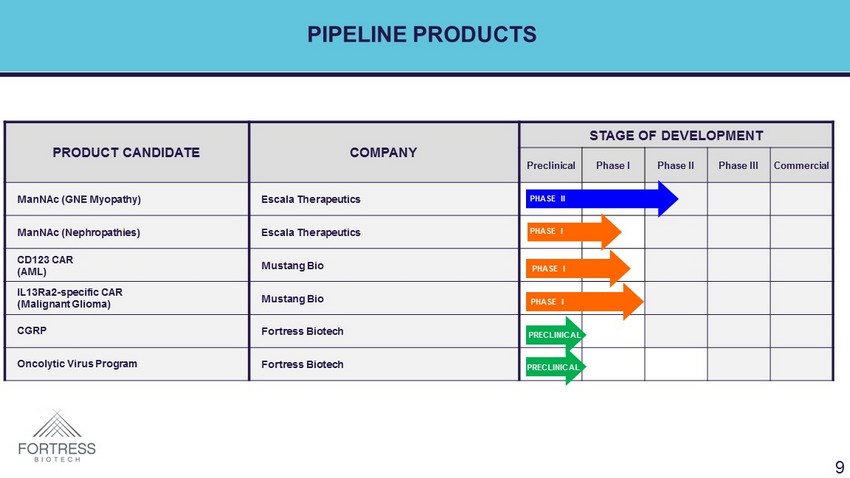
PIPELINE PRODUCTS PRODUCT CANDIDATE COMPANY STAGE OF DEVELOPMENT Preclinical Phase I Phase II Phase III Commercial ManNAc (GNE Myopathy) Escala Therapeutics ManNAc (Nephropathies) Escala Therapeutics CD123 CAR (AML) Mustang Bio IL13Ra2 - specific CAR (Malignant Glioma) Mustang Bio CGRP Fortress Biotech Oncolytic Virus Program Fortress Biotech PHASE II 9 PHASE II PHASE I PHASE I PHASE I PRECLINICAL PRECLINICAL

A FORTRESS BIOTECH COMPANY 10

GBM, A SIGNIFICANT UNMET MEDICAL NEED ~30,000 newly diagnosed GBMs annually in the US, Japan and five major EU markets GBM most aggressive form of brain tumor with extremely poor prognosis and overall survival (OS) Glioblastoma (GBM) is the most common primary malignant brain tumor • Median OS from diagnosis is ~15 months • Recurrent/Relapsed survival is ~5 - 7 months • 5 year survival of only 5% 11

CAR - T PROGRAM (MUSTANG BIOTECH) Chimeric Antigen Receptor (CAR) T Cell technology from City of Hope (COH) - Based on the research of Stephen Forman and Christine Brown, pioneers of CAR - T technology Research collaboration between Mustang and COH to identify additional CAR - T clinical candidates First two CAR - T’s in the clinic, targeting - IL13Ra2 - CD123(IL3) 12
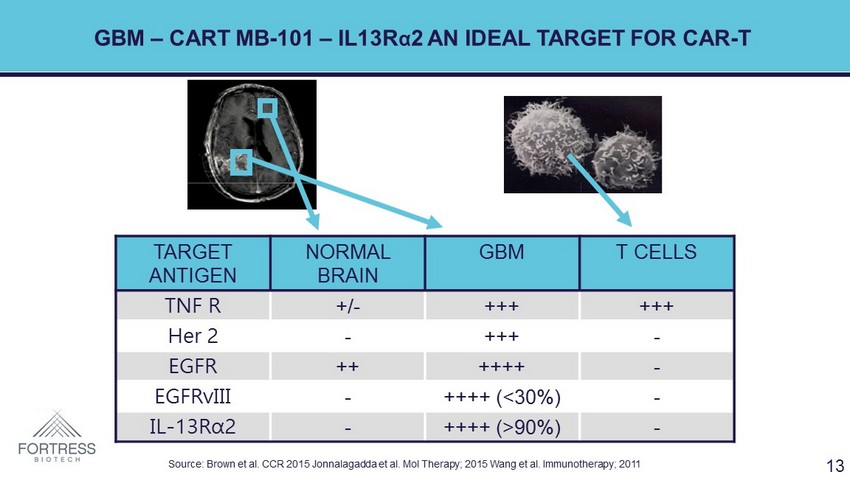
GBM – CART MB - 101 – IL13R α 2 AN IDEAL TARGET FOR CAR - T TARGET ANTIGEN NORMAL BRAIN GBM T CELLS TNF R +/ - +++ +++ Her 2 - +++ - EGFR ++ ++++ - EGFRvIII - ++++ (<30%) - IL - 13R α 2 - ++++ (>90%) - Source: Brown et al. CCR 2015 Jonnalagadda et al. Mol Therapy; 2015 Wang et al. Immunotherapy; 2011 13
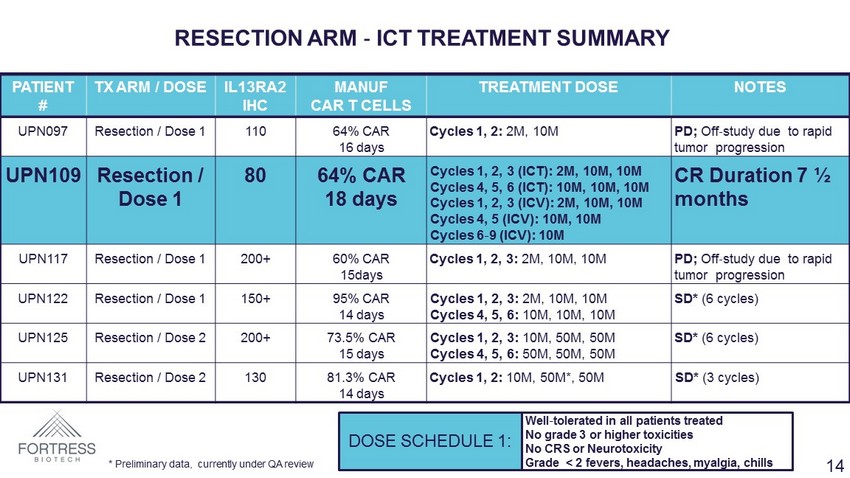
PATIENT # TX ARM / DOSE IL13RA2 IHC MANUF CAR T CELLS TREATMENT DOSE NOTES UPN097 Resection / Dose 1 110 64% CAR 16 days Cycles 1, 2: 2M, 10M PD; Off - study due to rapid tumor progression UPN109 Resection / Dose 1 80 64% CAR 18 days Cycles 1, 2, 3 (ICT): 2M, 10M, 10M Cycles 4, 5, 6 (ICT): 10M, 10M, 10M Cycles 1, 2, 3 (ICV): 2M, 10M, 10M Cycles 4, 5 (ICV): 10M, 10M Cycles 6 - 9 (ICV): 10M CR Duration 7 ½ months UPN117 Resection / Dose 1 200+ 60% CAR 15days Cycles 1, 2, 3: 2M, 10M, 10M PD; Off - study due to rapid tumor progression UPN122 Resection / Dose 1 150+ 95% CAR 14 days Cycles 1, 2, 3: 2M, 10M, 10M Cycles 4, 5, 6: 10M, 10M, 10M SD* (6 cycles) UPN125 Resection / Dose 2 200+ 73.5% CAR 15 days Cycles 1, 2, 3: 10M, 50M, 50M Cycles 4, 5, 6: 50M, 50M, 50M SD* (6 cycles) UPN131 Resection / Dose 2 130 81.3% CAR 14 days Cycles 1, 2: 10M, 50M*, 50M SD* (3 cycles) RESECTION ARM - ICT TREATMENT SUMMARY DOSE SCHEDULE 1: Well - tolerated in all patients treated No grade 3 or higher toxicities No CRS or Neurotoxicity Grade < 2 fevers, headaches, myalgia, chills * Preliminary data, currently under QA review 14
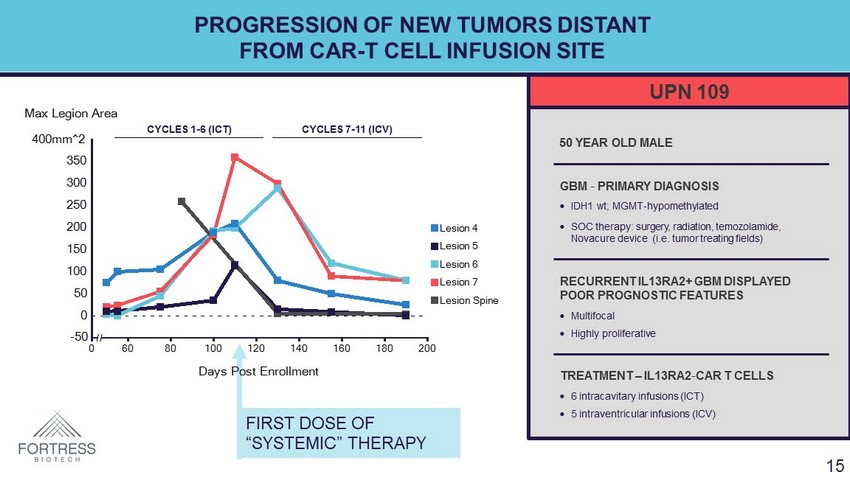
PROGRESSION OF NEW TUMORS DISTANT FROM CAR - T CELL INFUSION SITE 15 FIRST DOSE OF “SYSTEMIC” THERAPY -50 0 50 100 150 200 250 300 350 400mm^2 0 60 80 100 120 140 160 180 200 Days Post Enrollment Max Legion Area Lesion 4 Lesion 5 Lesion 6 Lesion 7 Lesion Spine UPN 109 50 YEAR OLD MALE GBM - PRIMARY DIAGNOSIS • IDH1 wt ; MGMT - hypomethylated • SOC therapy: surgery, radiation, temozolamide , Novacure device (i.e. tumor treating fields) RECURRENT IL13RA2+ GBM DISPLAYED POOR PROGNOSTIC FEATURES • Multifocal • Highly proliferative TREATMENT – IL13RA2 - CAR T CELLS • 6 intracavitary infusions (ICT) • 5 intraventricular infusions (ICV) CYCLES 1 - 6 (ICT) CYCLES 7 - 11 (ICV) 15
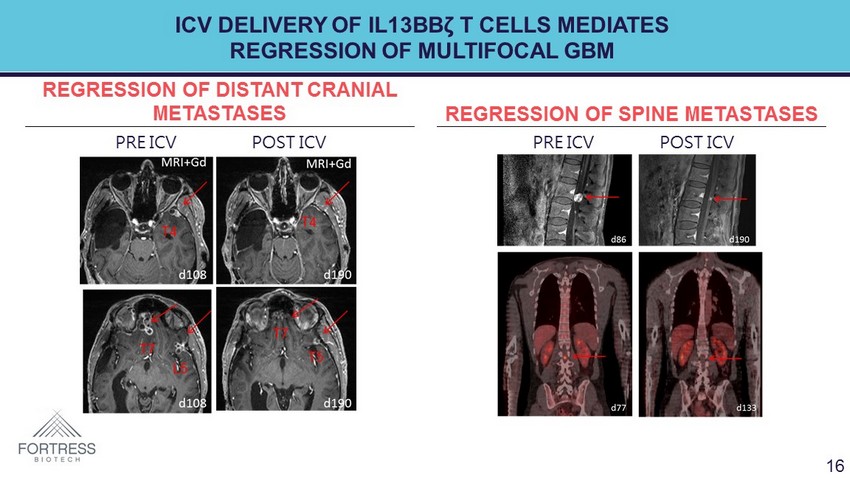
ICV DELIVERY OF IL13BB ζ T CELLS MEDIATES REGRESSION OF MULTIFOCAL GBM REGRESSION OF DISTANT CRANIAL METASTASES REGRESSION OF SPINE METASTASES PRE ICV POST ICV PRE ICV POST ICV 16
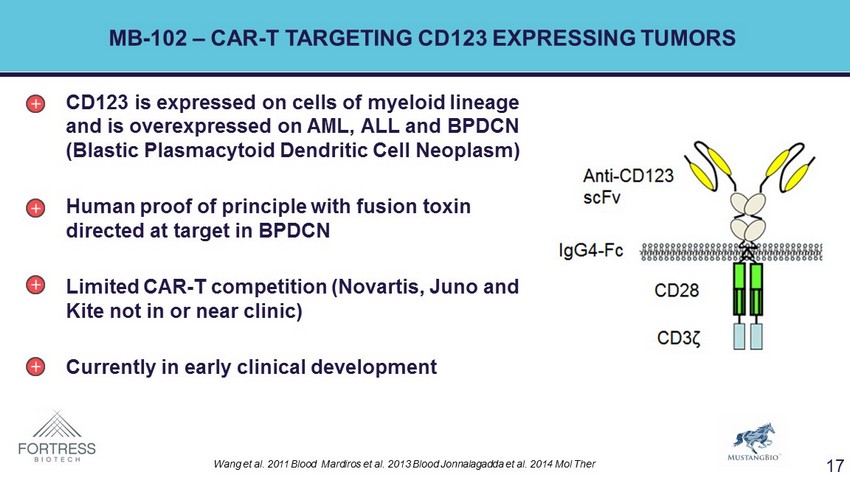
MB - 102 – CAR - T TARGETING CD123 EXPRESSING TUMORS CD123 is expressed on cells of myeloid lineage and is overexpressed on AML, ALL and BPDCN ( Blastic Plasmacytoid Dendritic Cell Neoplasm) Human proof of principle with fusion toxin directed at target in BPDCN Limited CAR - T competition (Novartis, Juno and Kite not in or near clinic) Currently in early clinical development Wang et al. 2011 Blood Mardiros et al. 2013 Blood Jonnalagadda et al. 2014 Mol Ther 17

A FORTRESS BIOTECH COMPANY 18
19 PEPVAX FOR POST - ASCT ( HELOCYTE ) • Antigen target applicable to ~35% of Patients • Healthy volunteer Phase 1 completed - Safe, well tolerated at all dose levels - Immunogenic • Phase 1b published in Lancet, Dec. 2015 • Multicenter Phase 2 in 96 Patients - Data by 1H2018 - NCI Funding: >$5M 19
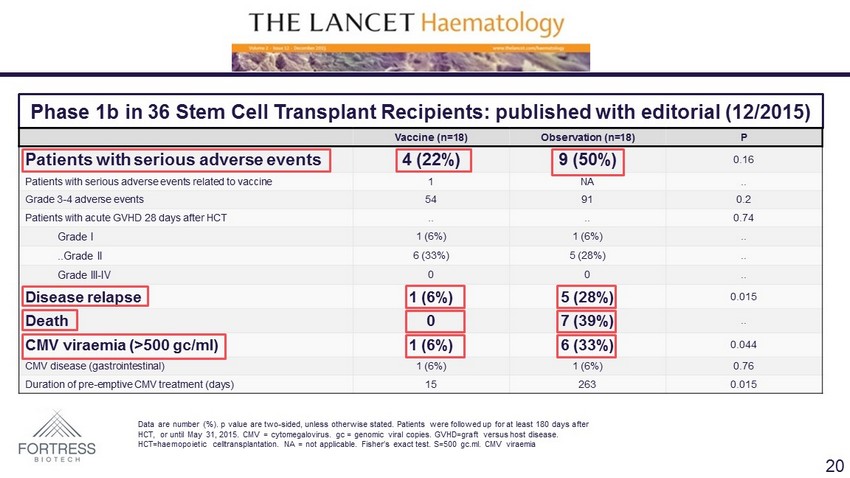
Phase 1b in 36 Stem Cell Transplant Recipients: published with editorial (12/2015) Vaccine (n=18) Observation (n=18) P Patients with serious adverse events 4 (22%) 9 (50%) 0.16 Patients with serious adverse events related to vaccine 1 NA .. Grade 3 - 4 adverse events 54 91 0.2 Patients with acute GVHD 28 days after HCT .. .. 0.74 Grade I 1 (6%) 1 (6%) .. ..Grade II 6 (33%) 5 (28%) .. Grade III - IV 0 0 .. Disease relapse 1 (6%) 5 (28%) 0.015 Death 0 7 (39%) .. CMV viraemia (>500 gc /ml) 1 (6%) 6 (33%) 0.044 CMV disease (gastrointestinal) 1 (6%) 1 (6%) 0.76 Duration of pre - emptive CMV treatment (days) 15 263 0.015 Data are number (%). p value are two - sided, unless otherwise stated. Patients were followed up for at least 180 days after HCT, or until May 31, 2015. CMV = cytomegalovirus. gc = genomic viral copies. GVHD=graft versus host disease. HCT= haemopoietic celltransplantation . NA = not applicable. Fisher’s exact test. S=500 gc.ml. CMV viraemia 20
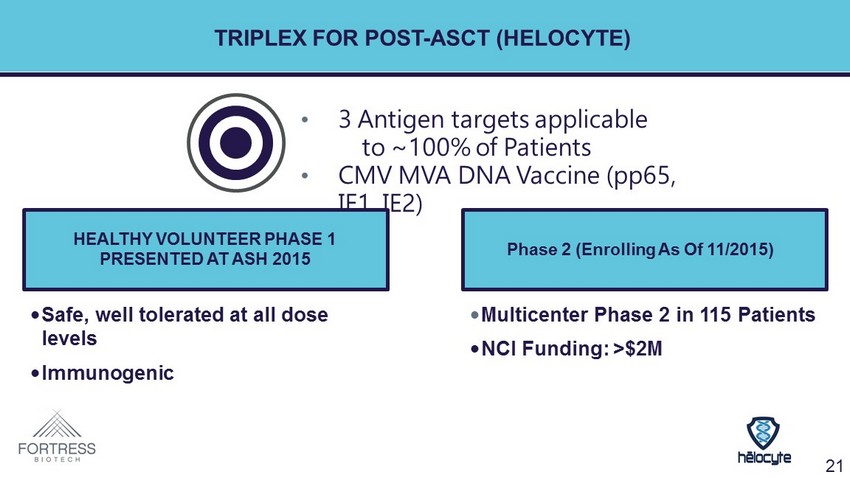
TRIPLEX FOR POST - ASCT ( HELOCYTE ) • 3 Antigen targets applicable to ~100% of Patients • CMV MVA DNA Vaccine (pp65, IE1, IE2) HEALTHY VOLUNTEER PHASE 1 PRESENTED AT ASH 2015 Phase 2 (Enrolling As Of 11/2015) • Safe, well tolerated at all dose levels • Immunogenic • Multicenter Phase 2 in 115 Patients • NCI Funding: >$2M 21

A FORTRESS BIOTECH COMPANY 22
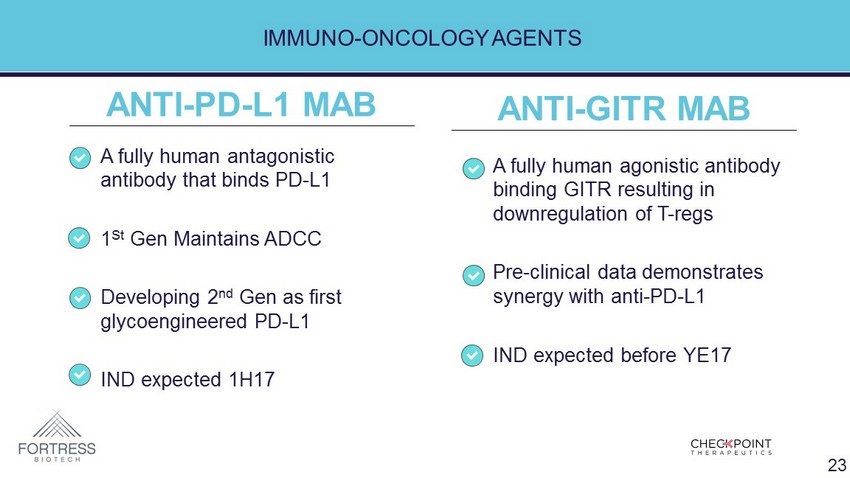
IMMUNO - ONCOLOGY AGENTS • A fully human agonistic antibody binding GITR resulting in downregulation of T - regs • Pre - clinical data demonstrates synergy with anti - PD - L1 • IND expected before YE17 • A fully human antagonistic antibody that binds PD - L1 • 1 St Gen Maintains ADCC • Developing 2 nd Gen as first glycoengineered PD - L1 • IND expected 1H17 ANTI - GITR MAB ANTI - PD - L1 MAB 23
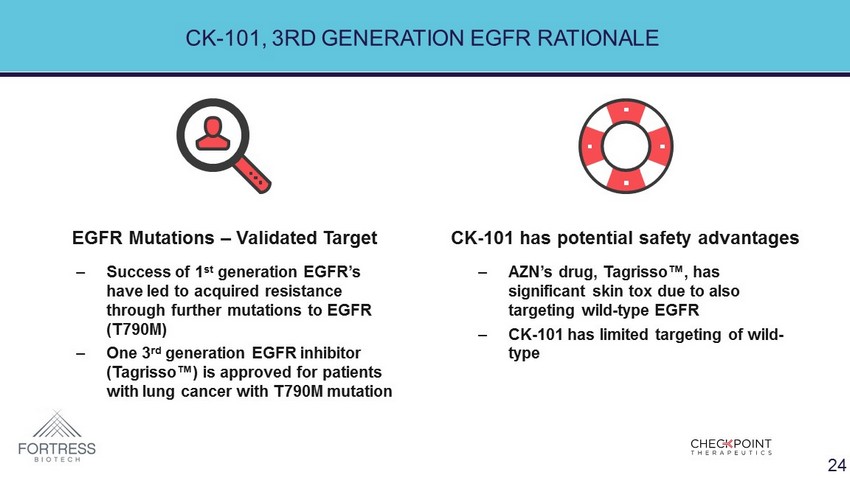
CK - 101, 3RD GENERATION EGFR RATIONALE ‒ Success of 1 st generation EGFR’s have led to acquired resistance through further mutations to EGFR (T790M) ‒ One 3 rd generation EGFR inhibitor ( Tagrisso ™) is approved for patients with lung cancer with T790M mutation ‒ AZN’s drug, Tagrisso ™, has significant skin tox due to also targeting wild - type EGFR ‒ CK - 101 has limited targeting of wild - type EGFR Mutations – Validated Target CK - 101 has potential safety advantages 24
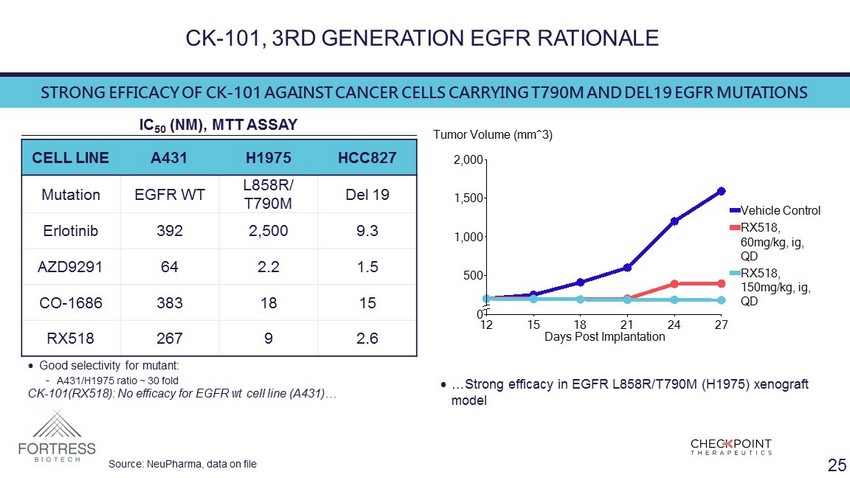
CK - 101, 3RD GENERATION EGFR RATIONALE 0 500 1,000 1,500 2,000 Tumor Volume (mm^3) 12 15 18 21 24 27 Days Post Implantation RX518, 150mg/kg, ig, QD RX518, 60mg/kg, ig, QD Vehicle Control CELL LINE A431 H1975 HCC827 Mutation EGFR WT L858R/ T790M Del 19 Erlotinib 392 2,500 9.3 AZD9291 64 2.2 1.5 CO - 1686 383 18 15 RX518 267 9 2.6 IC 50 ( NM ), MTT ASSAY • Good selectivity for mutant: - A431/H1975 ratio ~ 30 fold CK - 101(RX518): No efficacy for EGFR wt cell line (A431)… STRONG EFFICACY OF CK - 101 AGAINST CANCER CELLS CARRYING T790M AND DEL19 EGFR MUTATIONS • …Strong efficacy in EGFR L858R/T790M (H1975) xenograft model Source: NeuPharma , data on file 25
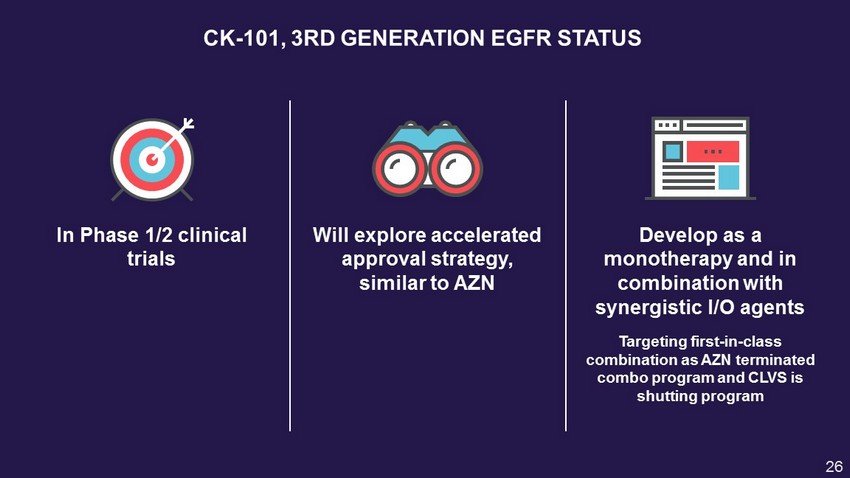
CK - 101, 3RD GENERATION EGFR STATUS In Phase 1/2 clinical trials Will explore accelerated approval strategy, similar to AZN Targeting first - in - class combination as AZN terminated combo program and CLVS is shutting program Develop as a monotherapy and in combination with synergistic I/O agents 26

A FORTRESS BIOTECH COMPANY 27
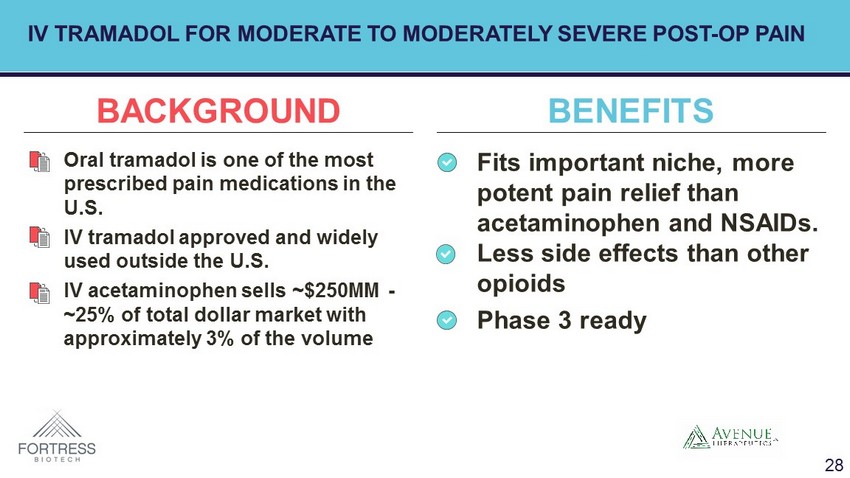
• Fits important niche, more potent pain relief than acetaminophen and NSAIDs. Less side effects than other opioids • Phase 3 ready • Oral tramadol is one of the most prescribed pain medications in the U.S. • IV tramadol approved and widely used outside the U.S. • IV acetaminophen sells ~$250MM - ~25% of total dollar market with approximately 3% of the volume BACKGROUND BENEFITS IV TRAMADOL FOR MODERATE TO MODERATELY SEVERE POST - OP PAIN 28
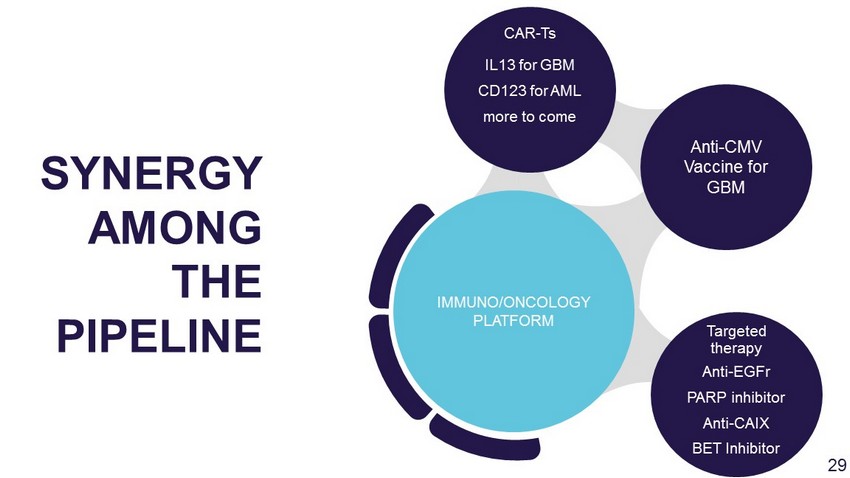
Anti - EGFr PARP inhibitor Anti - CAIX BET Inhibitor Anti - CMV Vaccine for GBM IL13 for GBM CD123 for AML more to come IMMUNO/ONCOLOGY PLATFORM SYNERGY AMONG THE PIPELINE CAR - Ts Targeted therapy 29
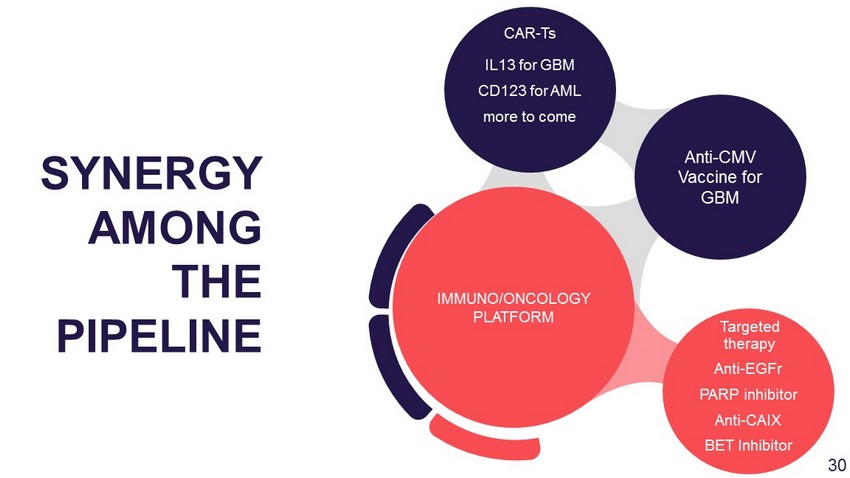
Anti - EGFr PARP inhibitor Anti - CAIX BET Inhibitor Anti - CMV Vaccine for GBM IL13 for GBM CD123 for AML more to come IMMUNO/ONCOLOGY PLATFORM SYNERGY AMONG THE PIPELINE CAR - Ts Targeted therapy 30
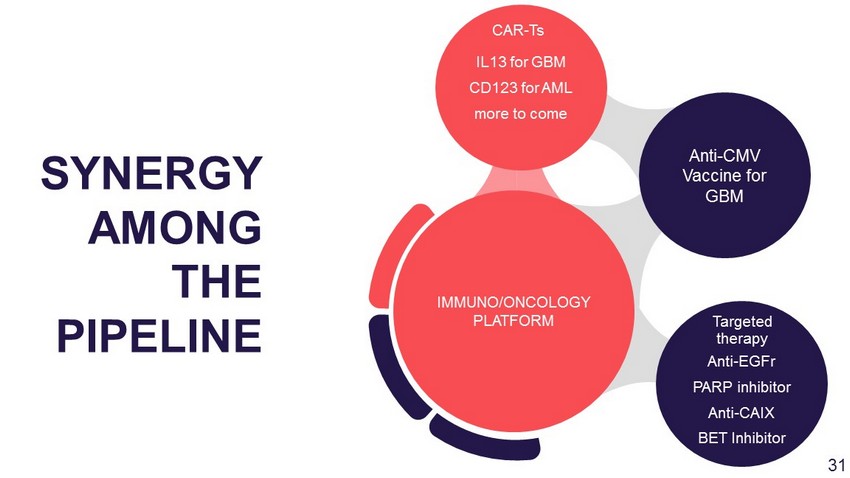
Anti - EGFr PARP inhibitor Anti - CAIX BET Inhibitor Anti - CMV Vaccine for GBM IL13 for GBM CD123 for AML more to come IMMUNO/ONCOLOGY PLATFORM SYNERGY AMONG THE PIPELINE CAR - Ts Targeted therapy 31
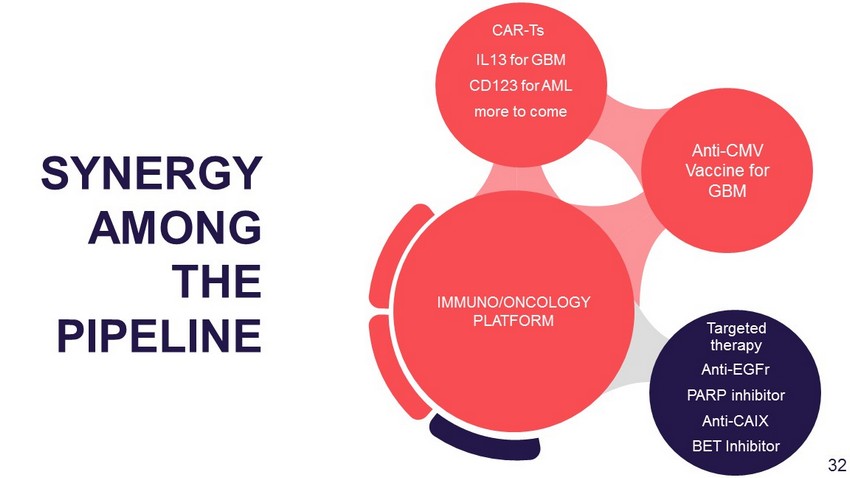
Anti - EGFr PARP inhibitor Anti - CAIX BET Inhibitor Anti - CMV Vaccine for GBM IL13 for GBM CD123 for AML more to come IMMUNO/ONCOLOGY PLATFORM SYNERGY AMONG THE PIPELINE CAR - Ts Targeted therapy 32
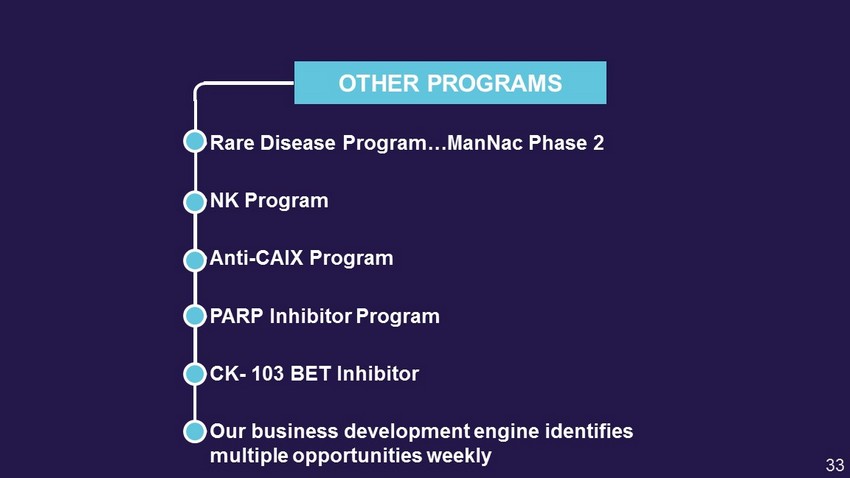
OTHER PROGRAMS • Rare Disease Program… ManNac Phase 2 • NK Program • Anti - CAIX Program • PARP Inhibitor Program • CK - 103 BET Inhibitor • Our business development engine identifies multiple opportunities weekly 33
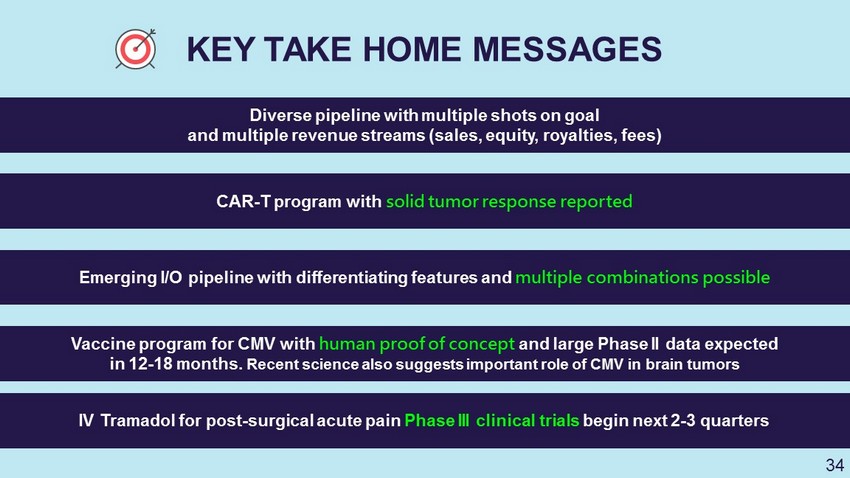
KEY TAKE HOME MESSAGES Diverse pipeline with multiple shots on goal and multiple revenue streams (sales, equity, royalties, fees) CAR - T program with solid tumor response reported Emerging I/O pipeline with differentiating features and multiple combinations possible Vaccine program for CMV with human proof of concept and large Phase II data expected in 12 - 18 months. Recent science also suggests important role of CMV in brain tumors IV Tramadol for post - surgical acute pain Phase III clinical trials begin next 2 - 3 quarters 34